45 eu language requirements for product labels
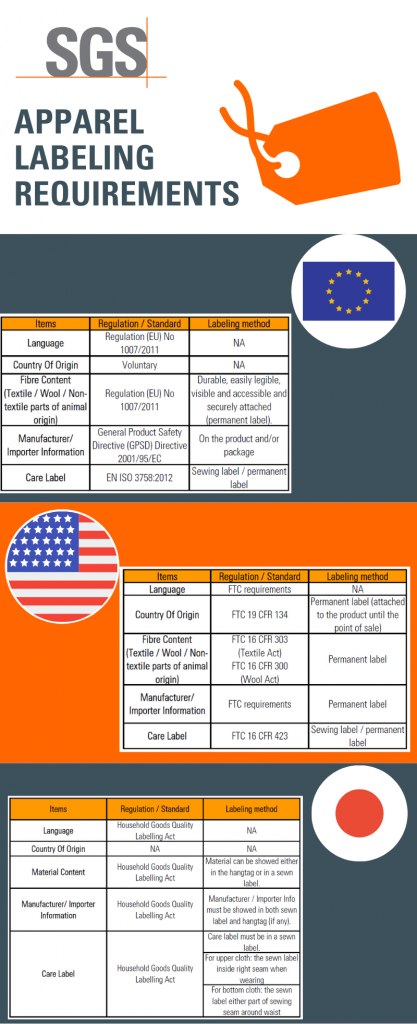
European Union Product Labelling Requirements: A Complete Guide EU Textiles Labelling Clothing and other products containing a minimum of 80% by weight of textile fibres must be labelled with the correct fibre composition (e.g. 100% Cotton or 100% Polyester). Further, the label must be permanent, which means it must either be attached to the clothing item or printed. A sticker is not enough. Product Examples How to Create a Label as per EU MDR 2017/745? A Standardized Symbol/Logo/ ICON must appear on all labels of the Product, indicating that the product being delivered into the Europe Union includes a medical device. The label must provide all the information needed for a customer to recognize the packaging's contents and intended use.
Cosmetic label requirements: Part II - COSlaw.eu For products imported from outside of the European Union, the labelling must specify the country of origin with the wording "made in". As per Article 60 of Regulation (EU) 952/2013 on the Union Customs Code, the country of origin is where the product has undergone the last substantial, economically justified processing or working.

Eu language requirements for product labels
2022 Label Guide: EU/CH Authorized Rep & UKRP - Casus Consulting February 21, 2022. Manufacturers need to consider the different labeling requirements for listing their in-country representatives in the EU, UK and Switzerland. The table below contains a high-level overview of the key requirements. Additional notes for the EU, United Kingdom and Switzerland are included below the table. EU MDR - Language requirements - omcmedical.com This information is required to be available in the Official languages of the Member states where the product is sold. This can be available either by electronic version nor printed version as per the requirement of the Economic operator or the user. The electronic version is covered by the EU regulation No.207/2012. EU IVDR Language Requirements & Languages in Each EU Member State On 26th May 2022, the In Vitro Diagnostic Regulation (IVDR) (EU) 2017/746 will become applicable across all 27 EU Member States, marking the start of a five-year progressive roll-out for in vitro diagnostic medical devices on the EU market. Among its various changes to the EU regulatory framework are the IVDR language requirements.
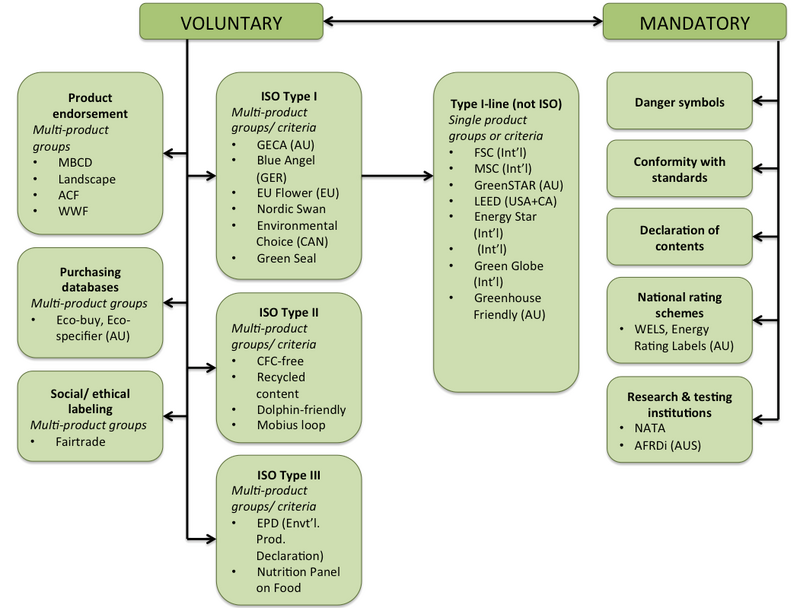
Eu language requirements for product labels. Sweden - Labeling/Marking Requirements - International Trade Administration Inspection and food labeling requirements were changed to conform to EU regulations when Sweden became a member of the EU on January 1, 1995. The first step in investigating the marking, labeling, and packaging legislation that might apply to a product entering the EU is to draw a distinction between what is mandatory and what is voluntary. Product compliance - ensure your product complies with EU rules - Your ... satisfy any traceability requirements: preserve the technical documentation and the EU declaration of conformity (for 10 years after the product is placed on the market or for the period specified for that product under EU law) give the product a type, batch or serial number for identification Switzerland - Labeling and Marking Requirements Generally, labeling and marking requirements follow EU regulations (CE labeling); however, a CE mark is not required for a product made only for Swiss domestic use. SECO coordinates the implementation of the Federal Act on Product Safety and more information can be found on their webpage on product safety (available in German, French, and Italian). Product-information requirements | European Medicines Agency EMA's guidance explains the content that should be included in these documents, as well as standard headings and the most commonly used standard statements and terms in all official European Union (EU) languages plus Icelandic and Norwegian, and defines the format and layout for the product information. EMA's guidance is without prejudice to:
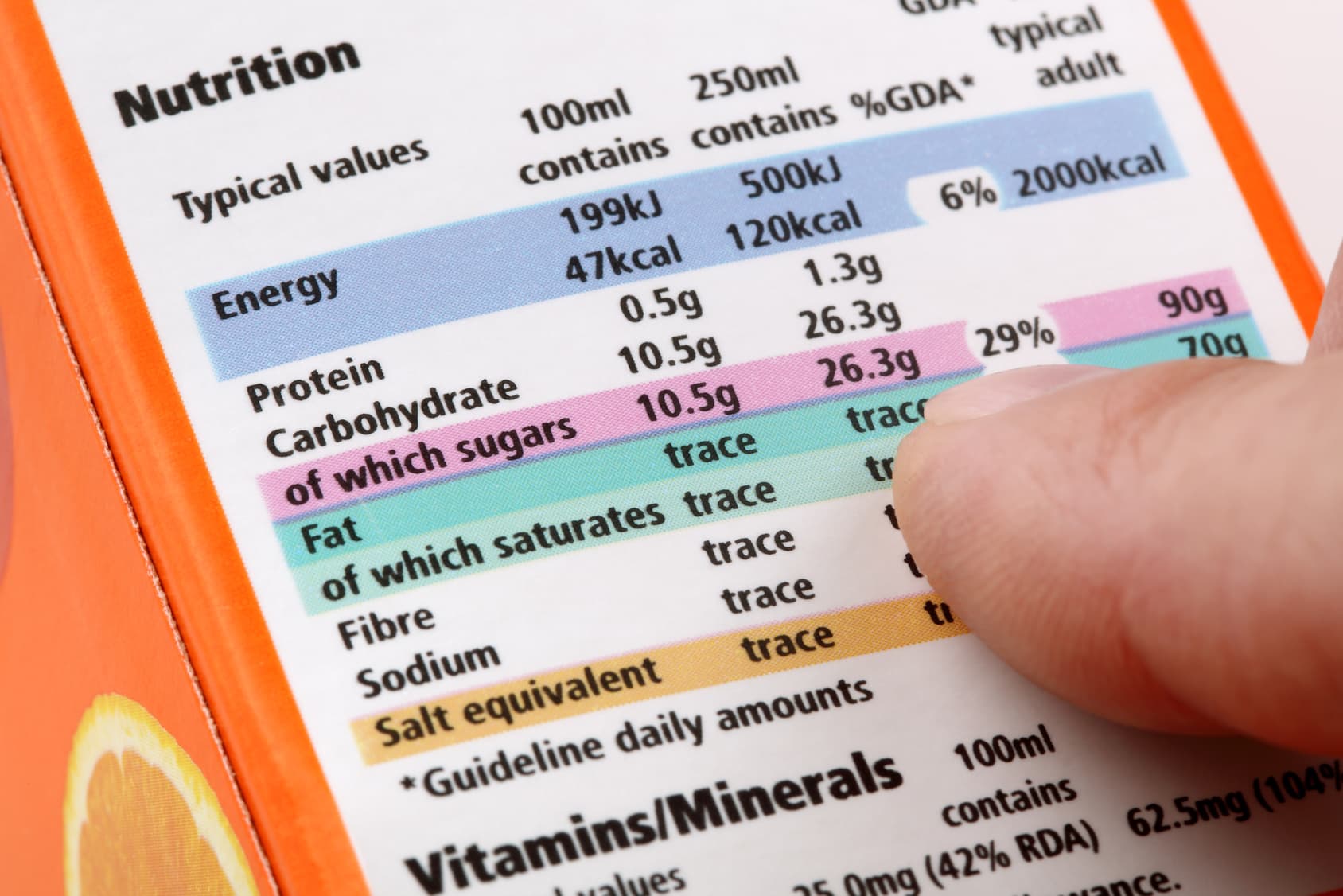
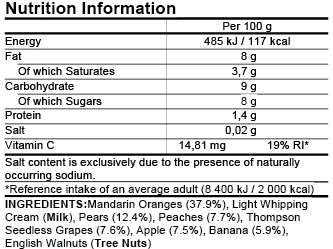
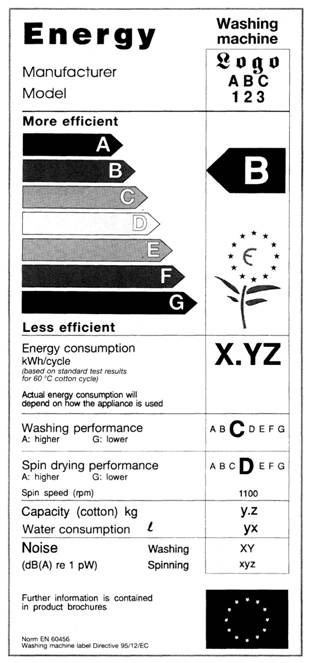
Product Labeling Regulations in the US, EU and Australia Here are few labeling requirements for imported products: a. The label must contain the identity of the prepackaged product in terms of its function or generic name. b. The label must contain the principal place of business and identity of the manufacturer or dealer. c. Food labelling - general EU rules - Your Europe Allergens - EU guidance Labelling Mandatory information must be printed using a font with a minimum x-height of 1.2 millimetres. If the largest surface area of packaging is less than 80 cm², you can use a minimum x-height of 0.9 mm. For packaging surface of less than 10 cm², you must list: name of the food United States Product Labeling Requirements: An Overview - Compliance Gate The label should be conspicuous, whether it is on the product or its packaging. The care label covers instructions may concern the following: Washing (e.g., "Machine wash") Drying (e.g., "Tumble dry") Ironing (e.g., "Iron") Bleaching (e.g., "Only non-chlorine bleach") Warning (e.g., "Wash with like colours") Product Regulations in the European Union: A Beginner's Guide Product regulations, such as safety standards and labeling requirements, are mostly 'harmonized' in the European Union. As such, the same regulations apply in all member states. A product that is compliant in the United Kingdom, is therefore also compliant in Poland and Italy.
EOF EU IVDR Language Requirements & Languages in Each EU Member State On 26th May 2022, the In Vitro Diagnostic Regulation (IVDR) (EU) 2017/746 will become applicable across all 27 EU Member States, marking the start of a five-year progressive roll-out for in vitro diagnostic medical devices on the EU market. Among its various changes to the EU regulatory framework are the IVDR language requirements. EU MDR - Language requirements - omcmedical.com This information is required to be available in the Official languages of the Member states where the product is sold. This can be available either by electronic version nor printed version as per the requirement of the Economic operator or the user. The electronic version is covered by the EU regulation No.207/2012. 2022 Label Guide: EU/CH Authorized Rep & UKRP - Casus Consulting February 21, 2022. Manufacturers need to consider the different labeling requirements for listing their in-country representatives in the EU, UK and Switzerland. The table below contains a high-level overview of the key requirements. Additional notes for the EU, United Kingdom and Switzerland are included below the table.





















Post a Comment for "45 eu language requirements for product labels"